Contact: +86 15677418852
Email: sales@hxgemstone.com
Synthetic Moissanite
Anew diamond imitation, synthetic moissanite (silicon carbide), is now being produced by C3 Inc. in near-colorless form for jewelry purposes. With refractive indices of 2.648 and 2.691, a dispersion of 0.104, a hardness of 9¼ on the Mohs scale, and a specific gravity of 3.22, synthetic moissanite is much closer to diamond in overall appearance and heft than any previous diamond imitation. The thermal properties of synthetic moissanite are also so close to those of diamond that the thermal probes currently on the market react to synthetic moissanite as if it were “diamond.” This new material can be readily separated from diamond on the basis of its anisotropic optical character, which produces a doubling in the appearance of facet junctions. A new instrument manufactured by C3 Inc. solely to distinguish synthetic moissanite from diamond was also examined for this study.
To the long list of diamond simulants currently available in the jewelry market, a new one has been added: synthetic moissanite. As typically happens with the introduction of a synthetic or simulant, there is considerable concern in the jewelry trade about this diamond imitation and its identification. One particular problem with synthetic moissanite is that its thermal properties are so close to those of diamond that it passes as “diamond” when tested with a thermal probe. This article reports on the examination of several samples of near-colorless synthetic moissanite (figure 1), both to characterize this material and to determine how it can be identified by standard gem-testing methods. The authors also evaluate a testing instrument developed by C3 Inc., which is intended to be used in conjunction with a thermal probe to distinguish this new simulant from diamond.
BACKGROUND
Diamond Imitations. All diamond imitations known to date have significant deficiencies. For example, synthetic spinel, colorless sapphire, and YAG (yttrium aluminum garnet) are much less brilliant than diamond. Synthetic rutile and strontium titanate are much too soft and display too much dispersion (“fire”). GGG (gadolinium gallium garnet) and CZ (cubic zirconia) have very high specific gravities, and the latter is somewhat brittle. Synthetic moissanite, by contrast, has gemological properties that are generally closer to those of diamond
Silicon Carbide. Since it was first manufactured a century ago, silicon carbide (SiC) has played an important industrial role as an abrasive. The growth of single crystals of silicon carbide has been studied for many years for two possible end uses: as a semiconductor material, and as a diamond substitute in jewelry. In fact, the promise of synthetic moissanite as a diamond imitation has been described several times in the gemological and related literature. Some of these publications included enthusiastic descriptions of faceted colored material (usually blue to green) and premature claims that Figure 1. Near-colorless synthetic moissanite is being marketed for jewelry purposes as a diamond imitation. The faceted pieces shown here, weighing from 0.09 to 0.57 ct, illustrate the appearance of this material. Because synthetic moissanite is doubly refractive, one can sometimes see doubling of the back facet junctions even with the unaided eye (particularly noticeable in the large round brilliant on the upper right). Photo © GIA and Tino Hammid.

colorless material was available (see, e.g., De Ment, 1948, 1949; Mitchell, 1962; McCawley, 1981). One of the authors (KN) noted the potential value of silicon carbide as a gem simulant 17 years ago. Referring to some pale tan to green to black centimeter-size crystals and faceted stones as large as half a carat, he stated that “these synthetic moissanites are quite attractive, and might provide a superb diamond imitation if they could only be made colorless” (Nassau, 1980, p. 253). At that time, however, a way had not been found to control either the color or the growth process to make synthetic crystals suitable for the gem industry.
Only recently, as described below, has the controlled growth of synthetic moissanite actually been achieved. Material that may appear near-colorless face-up in jewelry is now available for gemological use. It is being produced by Cree Research Inc. of Durham, North Carolina, and distributed by C3 Inc. A preliminary note on the new C3 material has appeared in this journal (Johnson and Koivula, 1996). Early work on silicon carbide was summarized by Mellor (1929; see also Powell, 1956). Edward G. Acheson (1893) appears to have been the first to recognize its hardness and potential as an abrasive. He made silicon carbide accidentally while trying to grow diamond by passing an electric arc between carbon electrodes through a mixture of carbon and molten clay (an aluminum silicate). He named the new substance “carborundum” (later to become a trade name). Subsequently, he obtained a better yield by using a mixture of carbon and sand. This same “Acheson” process is still used today in slightly modified form for the manufacture of silicon carbide for abrasive products (Divakar et al., 1993).
| Material | Mohs hardness | Toughness | R.I. | Birefringence | Dispersion | S.G. | Optic character | Pavilion flash colors |
|---|---|---|---|---|---|---|---|---|
| Diamond | 10 | Good to excellent | 2.417 | None | 0.044 moderate) | 3.52 | Singly refractive (isotropic) | Orange and blue on a few facets |
| Syn. moissanite | 9 1/4 | Excellent | 2.648, 2.691 | 0.043 (moderate) | 0.104 (strong) | 3.22c | Doubly refractive (uniaxial +) | Orange and blue |
| Syn. corundum | 9 | Excellent | 1.770,1.762 | 0.008–0.010(weak) | 0.018(weak) | 4.00 | Doubly refractive(uniaxial –) | Not diagnostic |
| Cubic zirconia | 8–8 1/2 | Good | 2.150–2.180 | None | 0.058 – 0.066(moderate) | 5.56–6.00 | Singly refractive (isotropic) | Orange over most of pavilion |
| Yttrium aluminum garnet (YAG) | 8 1/4 | Good | 1.833 | None | 0.028(weak) | 4.55 | Singly refractive(isotropic) | Blue, violet, some orange |
| Syn. spinel | 8 | Good | 1.728 | None | 0.020(weak) | 3.64 | Singly refractive(isotropic) | Blue over most of pavilion |
| Gadolinium gallium garnet (GGG) | 6 1/2 | Fair to good | 1.970 | None | 0.045(moderate) | 7.05 | Singly refractive(isotropic) | Blue, some orange |
| Syn. rutile | 6–6 1/2 | Poor to fair | 2.616,2.903 | 0.287(v. strong) | 0.330(v. strong) | 4.26 | Doubly refractive(uniaxial +) | Various spectral colors, widespread |
| Strontium titanate | 5–6 | Fair | 2.409 | None | 0.190(v. strong) | 5.13 | Singly refractive(isotropic) | Spectral colors, widespread |
chemist Henri Moissan discovered natural silicon carbide in the Canyon Diablo meteorite (Moissan, 1904). Kunz (1905) applied the name moissanite to the natural mineral in Moissan’s honor.
Since then, moissanite reportedly has been found in tiny amounts in other meteorites as well as in many terrestrial occurrences (e.g., Obukhov, 1972; Vigorova et al., 1978; Hallbauer et al., 1980; Moore et al., 1986; Rodgers et al., 1989; Mathez et al., 1995). Some or all of these occurrences may have been spurious, with the material derived from the older cutting wheels used to section mineral, rock, and meteorite specimens (Mason, 1962; Milton and Vitaliano, 1984; Milton, 1986), or from contamination by the huge amounts of silicon carbide produced industrially. (More than 36,000 tons were produced domestically, and 159,000 tons were imported into the United States, during the first seven months of 1997 [Balaziak, 1997].) However, the identification of moissanite as inclusions in diamond crystals before they were broken (Moore et al., 1986), and the determination of their abnormal isotopic composition (Mathez et al., 1995), confirm the occurrence of moissanite as a natural mineral.
The Structure of Moissanite. At first, considerable confusion resulted when investigators found a variety of different crystal structures for moissanite, including those having cubic (C), hexagonal (H), and rhombohedral (R) symmetries. This complexity results from the existence of polytypes, which represent different stacking sequences of hexagonal layers of atoms. More than 150 polytypes are known in the case of silicon carbide, all of which are properly designated as “moissanite” (Thibault, 1944; Ramsdell, 1947; Verma and Krishna, 1966).
At present, only the 4H and 6H polytypes of alpha-SiC can be grown in bulk form (i.e., as boules). Both polytypes are hexagonal, and both yield near-colorless material. The near-colorless synthetic moissanite described here is the 6H form. The 4H polytype, which has properties very close to those of the 6H polytype, is currently being produced for semiconductor uses, but not in near-colorless form. A key distinction from the 6H polytype is the much weaker absorption below 425 nm in the visible spectrum (Harris, 1995). Beta-SiC, which is the 3C polytype, is cubic and has a crystal structure even closer to that of diamond than the 4H or 6H polytypes. However, it cannot be grown in bulk form at present and it is inherently yellow (von Muench, 1982).
| Long-wave UV fluorescenceb | Absorption spectrum | Polish luster | Read-through effect | Relief in 3.32 S.G. liquid | Magnification |
|---|---|---|---|---|---|
| Inert or (usually) blue;sometimes yellow | “Cape” lines at 415 and 478 nm, sometimes no sharp lines | Adamantine | None | High | Included crystals, feathers, graining, bearding, naturals, waxy to granular girdle surface, sharp facet junctions |
| Inert to orange | Absorption below 425 nm; no sharp lines | Subadamantine | None | High | Doubling in appearance of facet junctions, whitish or reflective needles, rounded facet junctions, surface pits, polish lines |
| Inert | Not diagnostic | Vitreous to subadamantine | Very strong | Moderate | Gas bubbles, sharp facet junctions |
| Greenish yellow or yellowish orange | Not diagnostic | Subadamantine | Slight | Moderate | Gas bubbles, unmelted zirconium oxide powder, sharp facet junctions |
| Orange, sometimes inert | Not diagnostic | Subadamantine to vitreous | Strong | Low | Gas bubbles, sharp facet junctions |
| Weak green or inert | Not diagnostic | Vitreous to subadamantine | Very strong | Low | Gas bubbles |
| Pinkish orange or inert | Not diagnostic | Adamantine to vitreous | Moderate | Low | Gas bubbles, metallic platelets, rounded facet junctions, polishing marks |
| Inert | Absorption below 430 nm | Subadamantine to submetallic | None | High | Doubling in appearance of facet junctions,gas bubbles, rounded facet junctions,polishing marks |
| Inert | Not diagnostic | Vitreous to subadamantine | None | High | Gas bubbles, rounded facet junctions, polishing marks, scratches, abrasions |
Single Crystal (Bulk) Growth of Synthetic Moissanite.Growth techniques for silicon carbide have been studied for many decades (O’Connor and Smiltens, 1960; Verma and Krishna, 1966; Smoak et al., 1978; von Muench, 1984; Wilke, 1988; Davis et al., 1990; Divakar et al., 1993). Of these, only a seeded sublimation process, derived from the “Lely” approach, has proved viable for the controlled growth of large single crystals of synthetic moissanite (Davis et al., 1990; Nakashima et al., 1996).
In his original work, Lely (1955) used a cylinder, made of lumps of SiC, that had a cavity. This cylinder was heated in a sealed graphite crucible to 2500°C, at which point SiC crystals grew inside the cavity. Difficulties with controlling the chemical purity and the specific polytypes formed have led to many modifications. In particular, the use of carefully controlled atmospheres and temperature gradients, as well as the addition of a thin, porous graphite tube to line the cavity (which, through diffusion, provides improved control of the sublimation) have resulted in better control of the crystals that grow inside the tube. Many types of heating systems have been used, including radio frequency and resistance heating. Significant advances in the Lely process were reported in the USSR (Tairov and Tsvetkov, 1981).
The final breakthrough in the Lely process is revealed in a patent of Davis et al. (1990), where controlled growth of SiC occurs by sublimation from a feed powder, diffusion through graphite, and growth directly from the vapor phase on a seed crystal. With a sublimation process, the silicon carbide vaporizes and then recrystallizes without ever passing through a liquid stage. Details of this growth process as used for the near-colorless synthetic moissanite being distributed by C3 Inc. have not been released.
However, Davis et al. (1990) reported the growth of a 6H-polytype synthetic moissanite crystal (of gem quality, but not colorless) that was 12 mm in diameter and 6 mm thick during a six-hour growth period at the time of the initial patent filing in 1987. One of many recent papers in Nakashima et al. (1996), on various aspects of synthetic moissanite growth and its applications to the electronics industry by Cree Research, mentions the availability of 50-mm-diameter boules of 6H moissanite in 1994 (Tsvetkov et al., 1996). Such a boule could conceivably permit the manufacture of a brilliant-cut synthetic moissanite 50 mm in diameter and 28 mm high that would weigh about 380 carats! No production figures have been released on the amount of material that is currently available or could be made available for jewelry purposes. However, in December 1997, the senior author saw more than 1,000 faceted synthetic moissanites in the offices of C3 Inc. The company has stated that it plans to focus its marketing on near-colorless faceted material, with a release to the trade in early 1998, in a price range of 5%–10% of the average retail price of comparable diamonds (J. Hunter, pers. comm., 1997).
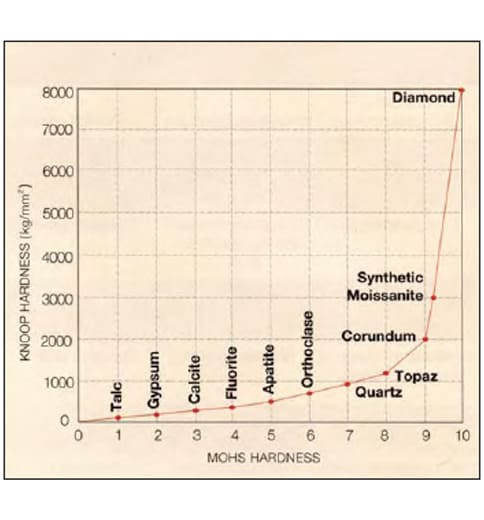
The hardness of synthetic moissanite is listed as 9¼ on the Mohs scale, which may be misleading. As shown in figure 2, the Mohs scale above a hardness value of 8 is disproportionately compressed when compared to a quantitative linear hardness scale such as that of Knoop (Bruton, 1978), which measures indentation hardness. The Knoop hardness of the 6H polytype of moissanite is reported to be in the range 2917–2954 kg/mm2 (2.91–2.95 Gpa) on the c crystal face (von Muench, 1984). The difference in Knoop hardness between corundum (Mohs 9) and moissanite (Mohs 91¼4) is larger than the difference between corundum and topaz (Mohs 8). Because of this disparity, synthetic moissanite cannot be polished by conventional techniques. The complex cutting process requires an additional, proprietary step (J. Hunter, pers. comm., 1997).
MATERIALS AND METHODS
The hardness of synthetic moissanite is listed as 9¼ on the Mohs scale, which may be misleading. As shown in figure 2, the Mohs scale above a hardness value of 8 is disproportionately compressed when compared to a quantitative linear hardness scale such as that of Knoop (Bruton, 1978), which measures indentation hardness. The Knoop hardness of the 6H polytype of moissanite is reported to be in the range 2917–2954 kg/mm2 (2.91–2.95 Gpa) on the c crystal face (von Muench, 1984). The difference in Knoop hardness between corundum (Mohs 9) and moissanite (Mohs 91¼4) is larger than the difference between corundum and topaz (Mohs 8). Because of this disparity, synthetic moissanite cannot be polished by conventional techniques. The complex cutting process requires an additional, proprietary step (J. Hunter, pers. comm., 1997).
In addition to the samples studied at GIA, one of the authors (KN) examined approximately 1,000 faceted synthetic moissanites and 100 preformed cubes at C3 Inc. The faceted samples included a 4.92 ct round brilliant, with a diameter of 11.9 mm and an approximate color grade of N, and a green round brilliant of 17.31 ct with a diameter of 17.5 mm. The faceted pieces studied at GIA were representative of this larger group of cut stones examined at C3 Inc. Also seen were 13 pieces of jewelry, which contained a total of 38 stones and included the ring discussed below
Standard gemological equipment used for all 23 main samples included a Gemolite Mark V binocular microscope, a polariscope, a desk-model spectroscope, and a short-wave (254 nm) and long-wave (365 nm) ultraviolet lamp. Approximate refractive index values were measured for 11 of these samples with a Jemeter Digital 90 infrared reflectometer. For research purposes only (the GIA Gem Trade Laboratory does not grade diamond simulants), trained graders used standard GTL procedures to try to assign diamond-equivalent color grades to the 18 samples that weighed more than 0.20 ct. Specific gravity measurements were obtained by the hydrostatic method on these same 18 samples, as well as the large piece of rough described above.
Because the thermal inertia ranges of diamond (0.55–1.7 cal/cm °C) and moissanite (1.6–4.8 cal/cm °C) overlap (Hoover, 1983; Harris, 1995), GIA researchers performed thermal inertia testing on the 23 core samples using a Ceres CZeckpoint, a Ceres Diamond Probe II, a GIA GEM DiamondMaster, and a GIA GEM Mini-DiamondMaster. For comparison, the following “colorless” gem materials were also tested using the same four instruments (with the number of samples in parentheses): diamond (types Ia [5 samples] and IIa [1]), sapphire (3), zircon (2), cubic zirconia (2), strontium titanate (2), synthetic spinel (2), synthetic rutile (7), yttrium aluminum garnet (2), gadolinium gallium garnet (2), “paste” (glass—2), and quartz (1). Thirteen colorless to yellow diamonds, with color grades ranging from E to Fancy Light Yellow, were also tested with the GIA Gem DiamondMaster. The senior author made thermal inertia measurements on 10 additional synthetic moissanites with a Ceres CZeckpoint, a Ceres Diamond Probe, a Presidium tester, and a Diamond Guard. Electrical conductivity of the 23 main synthetic moissanite samples was measured using a GIA GEM Instruments conductometer. The ultraviolet transparency of a diamond was compared to that of several synthetic moissanites in a specially designed viewing box, using a concept similar to that suggested by Yu and Healey (1980). X-ray transparency was recorded photographically using an HP Faxitron X-ray machine. Visual observation of the luminescence to X-rays of both materials (one diamond and 11 synthetic moissanites) was performed with a Picker portable X-ray unit adapted for this purpose.
Semiquantitative chemical analysis of eight of the synthetic moissanites examined at GIA was performed with a Tracor Spectrace 5000 energy-dispersive X-ray fluorescence (EDXRF) system. Several sets of excitation conditions were used to detect the widest possible range of chemical elements. Operating conditions yielded a lower limit of detection for transition metals of about 0.01 wt.%. Absorption spectra for eight of the GIA samples were obtained in the ultraviolet-visible range (250–750 nm) at room temperature using a Hitachi U4001 spectrophotometer, and in the infrared range using a Nicolet Magna 550 spectrometer. Raman spectra were recorded with a Renishaw System 2000 instrument. For details on any of these experimental procedures, please contact the GIA authors. A small instrument has been developed by C3 Inc. for the specific and limited purpose of distinguishing synthetic moissanite from diamond (see figure 3). Marketing of the Colorless Moissanite/ Diamond Tester Model 590 is expected to start early in 1998, at approximately the same time that synthetic moissanite is released to the jewelry trade (J. Hunter, pers. comm., 1997).
MATERIALS AND METHODS
The C3 tester determines relative transparency in the near-ultraviolet, where diamond transmits and synthetic moissanite absorbs. The accompanying instructions clearly state that this new instrument is to be used only in conjunction with a standard thermal inertia tester. Any unknown near-colorless gem should first be tested with a thermal inertia probe. Only those samples that the thermal inertia tester indicates are “diamond” would need to be tested with the C3 instrument, which would then identify whether any were synthetic moissanite. With the C3 instrument, the gem sample is illuminated at an angle by a small, high-intensity halogen lamp. Radiation from the lamp is transmitted and reflected within the sample, and the near-ultraviolet radiation that emerges from the table facet is detected by the instrument.
Use of the instrument is fairly simple. The operator brings the polished facet of a diamond or synthetic moissanite (loose or mounted) in contact with a fiber-optic probe that is located on the side of the instrument. As soon as the sample touches the probe, the instrument emits a sound (and an indicator light illuminates) if any material other than moissanite is detected; there is no sound or response from the indicator light if the item is synthetic moissanite. The synthetic moissanites, dia-monds, and other near-colorless gem materials listed above for the thermal probe testing were also tested with this instrument. In addition, the C3 instrument was used to test 13 pieces of jewelry containing synthetic moissanites, including the ring

RESULTS
Visual Appearance. The samples examined at GIA (reportedly representing the range of color that can be produced at present) were near-colorless to light yellow, green, and gray. Those samples that appeared to be in the I-to-K range all looked colorless in the face-up position, but around the L-to-N range they started to show face-up color. The one U-V stone was obviously gray face-up, which made it appear dark. Because many of these samples had grayish or greenish hues, it was difficult to arrive at an exact color grade on the traditional D-to-Z scale, which is comprised of predominantly yellowish stones. In the color grading of diamonds, grayish or greenish hues such as these fall outside normal procedures. However, when compared to diamonds that do fall on the D-to-Z scale, the 18 faceted synthetic moissanites above 0.20 ct ranged from the lower end of the “near-colorless” (G-to-J) category, with the best color being equivalent to I, to U-V in the “light” category of the scale. Synthetic moissan moissanites representing this approximate range of colors are illustrated
At first overall observation with the unaided eye, this material looks like a believable diamond imitation (see again figure 1). There were no eye-visible inclusions in any of the samples (on the diamond clarity grading scale, these synthetic moissanites would fall into the VVS to SI grades). They displayed no “read-through” effect, as can be seen in most other diamond imitations, which have lower refractive indices (see Hobbs, 1981). The samples showed moderate to high dispersion (greater than that of diamond or CZ, but less than that of synthetic rutile). Their brilliance appeared to be slightly less than that of diamond.









